Pharmaceutical Serialization Solutions
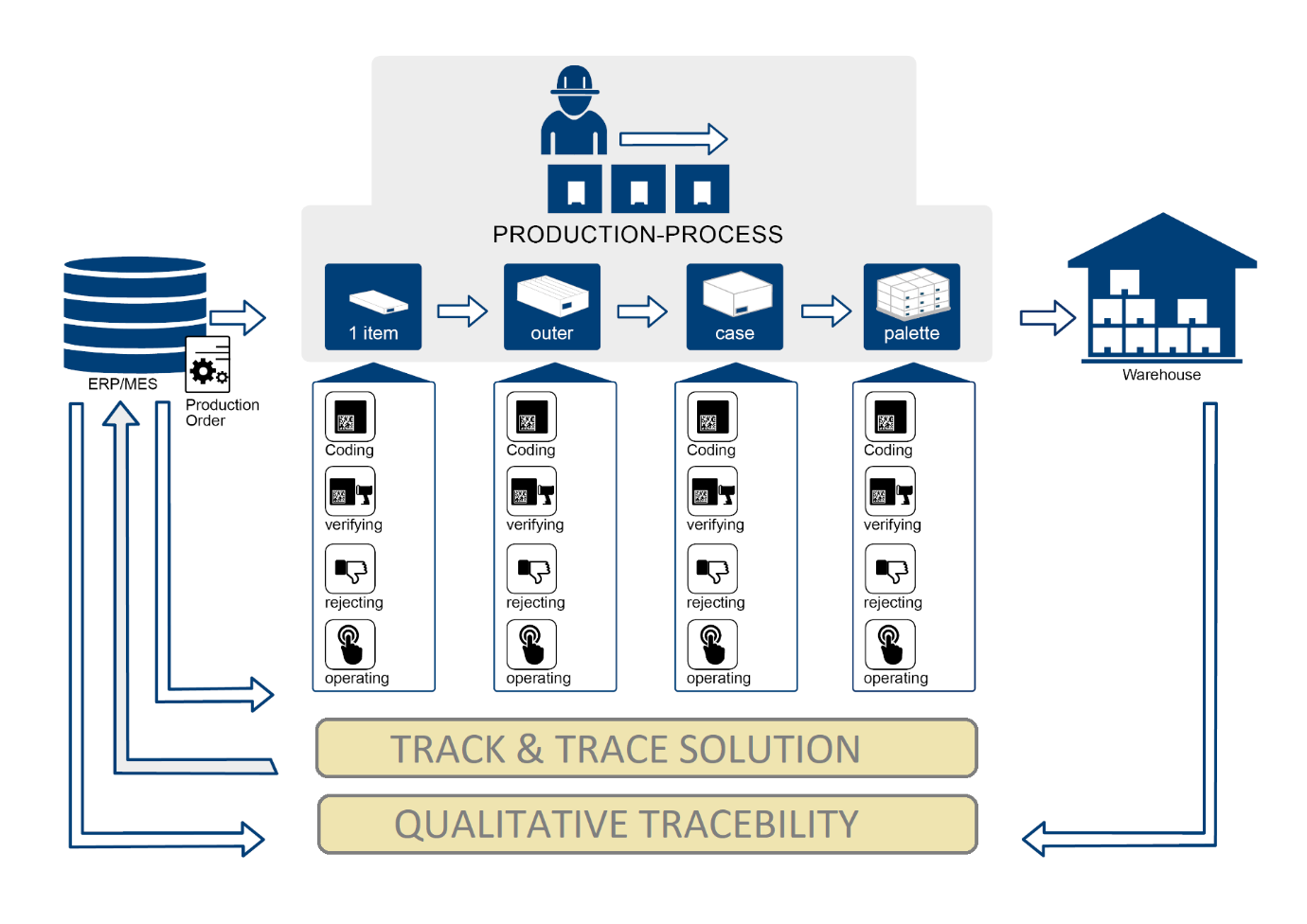
To help our customers with the requirement for compliance for pharmaceutical serialization, we offers a variety of options for track and trace serialization. By using our end-to-end software to track and trace pharmaceuticals, each and every package can be identified and monitored throughout the systems.
We have ability to track and trace from level of individual bottles and cartons, and to aggregate bottles and cartons to bundles or individual bottles or packaging to cases, to pallets. Our solution ensure we encode each blister package , bottle, and aggregate packaging, with expiration dates, GTINs, serial numbers, lot codes, and 2D matrix codes and GSI-Data Matrix symbols for complete compliance with all regulatory requirements.
Pharmaceutical Serialization Options:
- Aggregate individual units or bundles to cases
- Aggregate cases to pallets
- Rework of previously serialized products
- Individual bottles
- Individual cartons
- Aggregate bottles or cartons to bundles
Benefits of Pharmaceutical Serialization
- Encodes packages with expiration date, lot code, serial number, GTIN, and a 2D matrix code and a GSI-Data Matrix symbol
- Track products for anti-counterfeiting efforts
- Regulatory compliance
- Track and trace capability throughout the supply chain
- Consumer protection
- Brand protection
Pharmaceutical Serialization Options:
- Individual bottles
- Individual cartons
- Aggregate bottles or cartons to bundles
- Aggregate individual units or bundles to cases
- Aggregate cases to pallets
- Rework of previously serialized products
Our system interfaces with your system module to manages all of the data elements, work orders, and serial numbers and associated hierarchies required to support pharmaceutical serialization on the packaging line, serial number allocation, unit/case serialization, overall line data collection, alarming and reporting. Track and trace can be managed completely by this system.


Bottle Unit Serialization
Utilizing our printer stations, pre-encoded serialized labeled bottles will enter the Vision station and a 2D “helper” code can be printed on the bottle. Multiple vision systems will perform an inspection of the bottle label (expiration/lot/serial number/GTIN/2D matrix code) and code and associate the helper code to the label serialization code. Bottles will be ejected if they do not pass inspection. The helper code ensures proper aggregation of the bottles regardless of the print orientation.

Carton Unit Serialization
Utilizing our print and check stations, secondary packaging cartons are printed using our printers. Advanced machine vision systems inspect all the data printed on the carton (expiration/lot/serial number/GTIN/2D matrix code). Cartons will be ejected if they do not pass the inspection.

Bundle Aggregation
At our case packing stations, vision systems read and confirm all of the bottle helper codes or carton codes at the same time. Once the total number of bottles or cartons in the bundle are scanned and confirmed, the system will print code on the bundle.
Case Aggregation
At our bundle station, vision systems read and confirm all of the pharmaceutical serialization codes after the bottles or cartons (bundles or individuals) are placed into the case. Once the total number of bottles or cartons are scanned and confirmed, the system prints a case label from dedicated printers and the label is applied to the case.
. 
